Explore the intricate journey of pharmaceutical tablet development, from initial research and formulation to regulatory compliance and market launch. Learn about the crucial steps, challenges, and the continuous improvement cycle that shapes the creation of safe and effective pharmaceutical tablets.
- Home Posts tagged "continuous improvement"
Pharmacareer Analytical Testing, API selection, Clinical Trials, continuous improvement, drug formulation, healthcare, labeling, market launch, packaging, pharmaceutical industry, pharmaceutical tablet development, pre-formulation studies, process optimization, production, prototype development, quality control, regulatory compliance, scale-up, tablet formulation development slides for download
Pharmacareer Best Practices, Certification Processes, Compliance Training, continuous improvement, GMP Guidelines, Good Manufacturing Practices (GMP), industry standards, Manufacturing Excellence, Production Standards, quality assurance, quality control, regulatory compliance, Risk Mitigation, Safety Standards, Training Evaluation
Explore the world of Good Manufacturing Practices (GMP) and discover how these guidelines ensure the highest standards of quality and safety in production. From compliance training to rigorous certification processes, delve into the key aspects of GMP that contribute to manufacturing excellence and regulatory adherence.
Pharmacareer accurate data, avoiding mistakes, continuous improvement, cybersecurity, data integrity, health solutions, leadership role, medicine safety, Pharmaceuticals, regulatory compliance, technology in healthcare
Data integrity is the backbone of reliable information in various industries, ensuring that data is accurate, consistent, and unaltered throughout its lifecycle. In the context of pharmaceuticals, data integrity is particularly critical, impacting everything from research and development to manufacturing and regulatory compliance. This involves preventing and correcting errors in data, maintaining its quality, and safeguarding against unauthorized alterations. Whether it’s avoiding mistakes in dosage calculations or ensuring compliance with stringent regulatory standards, a commitment to data integrity is paramount for upholding the safety of products and the trust of consumers. Employing technologies like blockchain, automated validation processes, and stringent quality controls, industries strive to minimize risks, promote transparency, and foster a culture of continuous improvement in data management practices. In essence, data integrity is not just a regulatory requirement; it’s a fundamental aspect of delivering accurate, safe, and high-quality products and services.
Pharmacareer audit preparation, audit process, clinical CRO audits, clinical research, Compliance, continuous improvement, corrective actions, data integrity, ethics in research, GCP, investigator training, quality assurance, Regulatory guidelines, trial management, vendor oversight
Embark on a comprehensive journey of clinical CRO audits with our 100-question guide. Uncover the intricacies of trial management, data integrity, and compliance. Learn how to conduct effective audits, foster collaboration, and drive continuous improvement. Explore the essential steps for a successful audit process and ensure the excellence of clinical research endeavors. Access the complete guide now.”
Pharmacareer Compliance, continuous improvement, corrective actions, deviation assessment, deviation control, deviation management, deviation reporting, preventive actions, Product Quality, root cause analysis, SOP
Learn how to effectively manage deviations from established processes with this comprehensive Standard Operating Procedure (SOP) Deviation Control. Understand the procedures for deviation identification, assessment, investigation, and resolution to ensure product quality, compliance, and continuous improvement within your organization.
Pharmacareer Acceptable Quality Level, AQL, continuous improvement, customer expectations, decision-making, defects reduction, manufacturing processes, Product Quality, sampling procedures
Learn about Acceptable Quality Level (AQL) and its importance in maintaining consistent product quality. This guide covers AQL establishment, sampling procedures, decision-making, and continuous improvement for optimal results. Understand how AQL contributes to reducing defects and meeting customer expectations.
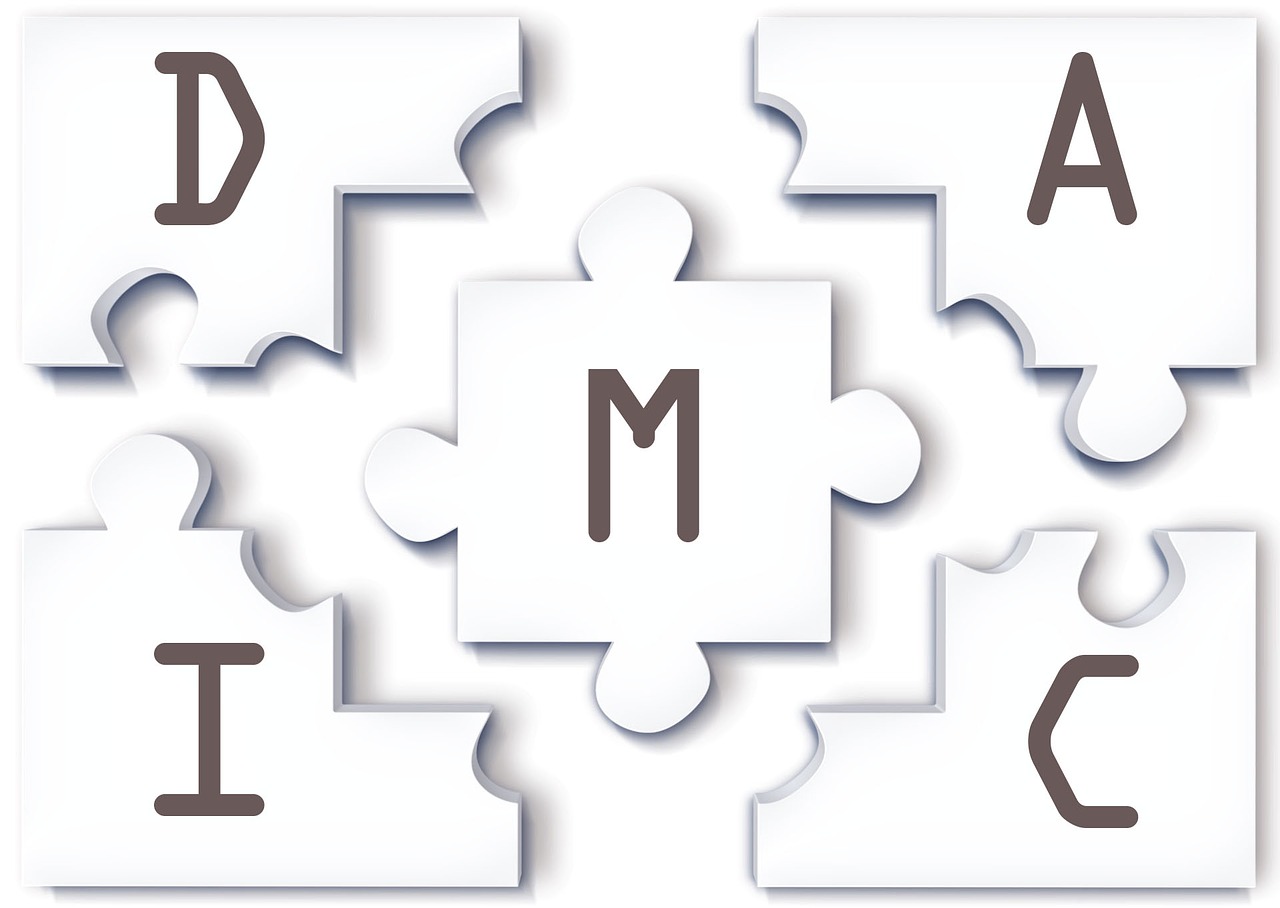
Pharmacareer ASQ Certification, Belts in Six Sigma., Black Belt, Certification Types, Champion, continuous improvement, CTQ Tree, data analysis, Design for Six Sigma, DFSS, DMAIC, FDA guidance, Green Belt, IASSC Certification, Leadership in Six Sigma, lean, Lean Six Sigma, Master Black Belt, Pharmaceuticals, Problem-Solving, process improvement, Project Management, Quality Management, root cause analysis, Six Sigma, Six Sigma Levels, Statistical Tools, White Belt, Yellow Belt
“Explore the comprehensive guide to Six Sigma methodologies, including DMAIC, CTQ Tree, and Root Cause Analysis. Learn about the various Six Sigma certification levels – White Belt, Yellow Belt, Green Belt, Black Belt, Master Black Belt, and Champion. Discover the integration of Lean principles and Design for Six Sigma (DFSS). Gain insights into the certification process offered by ASQ and IASSC. Elevate your understanding of quality management, process improvement, and data-driven decision-making in the world of Six Sigma.”